The team of the Haematology Department of the Marqués de Valdecilla Hospital–IDIVAL, with Dr. Arancha Bermúdez as principal investigator, has participated in a leading international clinical trial on the treatment of chronic graft-versus-recipient disease (cGVHD). The results have recently been published in the prestigious New England Journal of Medicine.
CGRID is a serious, long-term complication affecting patients undergoing allogeneic haematopoietic transplantation, and is the leading cause of non-relapse morbidity and mortality after the procedure. This disease, whose prevalence is increasing due to the increase in transplantation and its application in older patients, can affect multiple organs such as the skin, liver, lungs and digestive system, among others.
The study, involving 50 hospitals in the Americas, Europe and Asia, investigated the efficacy of axatilimab, a new humanised monoclonal antibody that works by inhibiting the CSFR1 receptor on macrophages and monocytes, cells involved in the development of cRTI. This new therapeutic approach offers a promising alternative for patients who do not respond to standard therapies.
The results are encouraging: 74% of patients treated with axatilimab responded positively, achieving early improvement. Although long-term follow-up is required, these data suggest new hope for patients who, until now, lacked viable therapeutic options.
Dr Bermúdez stresses the importance of this type of research, which offers new opportunities for patients severely affected by post-transplant complications. Advances in immunotherapies such as axatilimab open a door to significantly improve the quality of life of many patients.
For more information, the full article can be found in the New England Journal of Medicine here.
HUMV-IDIVAL participates in a pioneering treatment for chronic graft-versus-host disease
The team of the Haematology Department of the Marqués de Valdecilla Hospital–IDIVAL, with Dr. Arancha Bermúdez as principal investigator, has participated in a leading international clinical trial on the treatment of chronic graft-versus-recipient disease (cGVHD). The results have recently been published in the prestigious New England Journal of Medicine. CGRID is a serious, long-term complication […]
Next Friday, 29 September, from 17:00 hours, IDIVAL (Valdecilla Health Research Institute) will be present at the European Researchers’ Night, which will be held in Plaza Pombo in Santander. This scientific dissemination event, organised by the University of Cantabria, will bring together more than 100 researchers from various institutions to bring science closer to society in a dynamic and accessible way.
IDIVAL will have more than 30 researchers at stand 21, entitled ‘Moving towards personalised medicine’, where visitors will be able to learn first-hand about the work in different fields of health and science. The research groups that will participate are:
- Cohorte Cantabria: ‘Be part of health research’, with studies on the population and its impact on personalised medicine.
- Cardiology research group: ‘Discover how your heart works’, with information on the mechanisms and technologies that study cardiovascular health.
- Mental illness research group: ‘Discover how your brain reacts to different stimuli’, exploring the brain and its responses.
- Psychiatry and mental health research group: ‘Discover how auditory hallucinations are experienced’, presenting research on perception and psychiatric disorders.
- Cell signalling and therapeutic targets in cancer (IDIVAL) and Optics (UC): ‘Is it or is it not cancer? The answer is in the light’, showing how optics can improve oncological diagnoses.
This event, with free admission, is an excellent opportunity to discover the advances in personalised medicine and science, and how they impact on people’s lives. Don’t miss it!
Check out the full programme of the event here.
IDIVAL participates in the European Researchers’ Night with the stand “Moving towards personalised medicine”.
Next Friday, 29 September, from 17:00 hours, IDIVAL (Valdecilla Health Research Institute) will be present at the European Researchers’ Night, which will be held in Plaza Pombo in Santander. This scientific dissemination event, organised by the University of Cantabria, will bring together more than 100 researchers from various institutions to bring science closer to society […]
The Valdecilla Health Research Institute (IDIVAL) has recently signed a collaboration agreement with the Universal Accessibility Foundation (FAU), with the aim of promoting projects that promote inclusion and accessibility in the field of health. This agreement reflects the values shared by both institutions, such as the commitment to equity, accessibility and the continuous improvement of health services for all people, regardless of their abilities.
The FAU, known for its work in favour of the inclusion of people with disabilities and the elimination of architectural, physical and social barriers, finds in IDIVAL a strategic ally in research to develop innovative solutions that improve accessibility in the healthcare environment. This commitment to equality and inclusion is a fundamental pillar for both entities.
The agreement was signed by Galo Peralta, Managing Director of IDIVAL, and Raúl San Miguel, President of the FAU, in an act that reaffirms the collaboration between the health and social sectors to ensure accessible and quality care.
This agreement underlines the interest of both organisations in developing projects that enable progress in the creation of more inclusive and accessible healthcare environments for all, thus promoting universal healthcare that responds to the needs of each individual.
More information about the UAF: https://www.fundaver.es/
IDIVAL and the Universal Accessibility Foundation sign agreement to promote inclusive health research
The Valdecilla Health Research Institute (IDIVAL) has recently signed a collaboration agreement with the Universal Accessibility Foundation (FAU), with the aim of promoting projects that promote inclusion and accessibility in the field of health. This agreement reflects the values shared by both institutions, such as the commitment to equity, accessibility and the continuous improvement of […]
Cohorte Cantabria adds a new ally in its challenge to reach 50,000 participants. The municipalities furthest from the arc of the Bay of Santander are now the main target
On 17 September at the IDIVAL facilities, a collaboration agreement was formalised with Sidenor, a company that produces special long steels with a low carbon footprint and a proven social and environmental commitment.
Sidenor is a large Spanish company that actively collaborates with suppliers, social entities, training centres and sports and cultural associations in the regions where it is located. In this case, the Reinosa plant will join Cohorte Cantabria, disseminating the project in its networks and encouraging its workers to participate.

The agreement was signed by Sara Jainaga, Sustainability Director of Sidenor, together with Dr. Galo Perlata, Managing Director of IDIVAL. The meeting was also attended by part of the managers of the Reinosa plant, Emilio Fernández Lucio, and Beatriz De la Torre, HR Director, as well as Dr. Marcos López Hoyos, Scientific Director of IDIVAL and Cohorte Cantabria.
We are very fortunate to have companies so committed to research in our region!
More information about Sidenor on their website.
IDIVAL and Sidenor join forces to boost health research: The Reinosa plant joins the Cohorte Cantabria project
Cohorte Cantabria adds a new ally in its challenge to reach 50,000 participants. The municipalities furthest from the arc of the Bay of Santander are now the main target On 17 September at the IDIVAL facilities, a collaboration agreement was formalised with Sidenor, a company that produces special long steels with a low carbon footprint […]
From September 9 to 12, 2024, the online course “State of the Art in Rhinology” was held, coordinated by Dr. Jaime Viera, Assistant Physician of the Otorhinolaryngology Service of the Marqués de Valdecilla University Hospital. This course, broadcasted through the Zoom platform, had almost 400 participants from 22 countries and was organized by IDIVAL, the Ministry of Health of the Government of Cantabria and the University of Cantabria. This forum was endorsed by SCReN, SEORL CCC, the Spanish Society of Clinical Pharmacology and the ITEMAS and Biobanks and Biomodels platforms of the Carlos III Health Institute, and was sponsored by Sanofi and GSK. This program has been accredited with 1.6 CFC credits from the National Health System.
The event brought together highly prestigious national and international speakers, who addressed the latest advances in surgery and medical treatments related to rhinology. Among the highlights were Dr. Adriana Izquierdo from Clínica Teknon in Barcelona, Dr. Eduardo Morera from Hospital Universitario Son Espases, and Dr. Isam Alobid, President of the Rhinology Commission of the SEORL. Renowned international specialists also participated, such as Dr. Raymond Kim from the University of Auckland (New Zealand) and Dr. Alkis J. Psaltis from the University of Adelaide (Australia).
During the conference, participants had the opportunity to attend sessions covering a wide variety of current topics, such as the evolution in the diagnosis and treatment of chronic sinusitis, innovations in the surgery of nasosinus tumors and the latest techniques in nasal reconstruction and treatment of the sense of smell. Dr. Manuel Bernal-Sprekelsen, President of the SEORL, opened the course with a session on the history of rhinology and its evolution up to the present day. Another relevant session was given by Dr. Beatriz Abascal, who dealt with the use of biological drugs in the treatment of the airway.
Throughout the conference other topics have been discussed, such as the session led by Dr. Juan Maza who presented the advances in the surgical management of maxillary sinusitis or the intervention of Dr. Raymond Kim who delved into the microbiological differential diagnosis of this condition. These presentations were followed by different debates moderated by Dr. Jaime Vieira, Dr. Javier Ospina or the one led by Dr. David Lobo, in which best practices and new surgical techniques in the multidisciplinary management of odontogenic sinusitis were discussed.
The course concluded on Thursday, September 12, with a session dedicated to olfaction and nasal reconstruction, moderated by Dr. Isam Alobid, highlighting topics such as septal perforations and olfactory training, presented by Dr. Adriana Izquierdo, and functional rhinoplasty by Dr. Eduardo Morera.
This forum has proven to be a key training reference for otolaryngologists and other medical professionals interested in rhinological pathologies. Discussions on the implementation of new techniques and multidisciplinary collaboration to address clinical challenges have consolidated the importance of this type of event in the advancement of medical knowledge.
Rhinology refresher course successfully concluded with the participation of international experts
From September 9 to 12, 2024, the online course “State of the Art in Rhinology” was held, coordinated by Dr. Jaime Viera, Assistant Physician of the Otorhinolaryngology Service of the Marqués de Valdecilla University Hospital. This course, broadcasted through the Zoom platform, had almost 400 participants from 22 countries and was organized by IDIVAL, the […]
The joint action “Health4EUkids” of the European project EU4Health, co-financed by the European Union and HaDEA, is framed in the field of health promotion and prevention of non-communicable diseases. This action, led in Cantabria by the Health Economics Group, the Clinical and Translational Research Group in Digestive Diseases, the Pediatrics Service of the Marqués de Valdecilla Hospital and collaborators of the Cantabrian Health Service, highlights best practices and specific risk factors identified from previous research results on childhood obesity, developed in previous initiatives such as “Grünau Moves” (Germany) and “Smart Family” (Finland).
At the WP5 & WP6 meeting of the European project “Health4EUkids” to be held on September 19-20, 2024 in Menorca (Spain), the General Directors of Public Health of the Ministry of Health, Mr. Pedro Gullón, Conselleria de Sanidad de la Comunidad Valenciana, Ms. Ruth Usó, and the Conselleria de Sanidad de la Comunidad Valenciana, Ms. Ruth Usó, and the Conselleria de Salud de la Comunidad Valenciana, Ms. Ruth Usó, will be present at the meeting. Ruth Usó, and the Conselleria de Salut of the Balearic Islands, Ms. Antonia Elena Esteban Ramis will welcome the collaborating partners (Belgium, Croatia, Greece, Finland, Hungary, Italy, Lithuania, Malta, Poland, Portugal, Slovenia, Spain).
On the first day of the meeting, Apostolos Vantarakis (UPAT, Greece) will chair Session 1: Overview of WP1 coordination and strategic direction, and Rosana Peiró (Fisabio & GDPH Valencia, Spain) and Heli Kuusipalo (THL, Finland) will chair Session 2: Meeting overview – Agenda highlights and objectives. Päivi Mäki (THL, Finland) will present the common points of WP5 & WP6: “Monitoring the prevalence of childhood overweight and obesity – Opportunities, challenges and situation update”, while Carmen Barona (DGSP-GVA, Spain) will present the “Regional Monitoring System on Childhood Obesity and Overweight, DGSP-Region of Valencia”. Finally, in Session 3: Presentation of Implementation Projects in WP5 & WP6, each partner will present the follow-up of the WP5 & WP6 Pilots, Barriers and Challenges.
The second day of the meeting will start with the follow-up of the WP5 & WP6 Pilots, Barriers and Challenges. This will be followed by Session 4: Evaluation Indicators – Assessing Progress and Measuring Impact, with Peter Csizmadia (NNGYK, Hungary) presenting the evaluation indicators for WP5 & WP6 followed by workshops on evaluation and adaptation, with parallel sessions for WP5 and WP6. Finally, Evaluation Proposals – Sharing ideas between WP5 and WP6 and Concluding Remarks and next steps will be chaired by Rosana Peiró (Fisabio & GDPH Valencia, Spain) and Heli Kuusipalo (THL, Finland).
For more information about the project, you can consult the Health4EUkids website and its social networks (Twitter, Linkedin, Instagram, Facebook).

IDIVAL participates in the “Health4EUkids” Project meeting: WP5 “Grunau Moves” & WP6 “Smart Family” in Menorca
The joint action “Health4EUkids” of the European project EU4Health, co-financed by the European Union and HaDEA, is framed in the field of health promotion and prevention of non-communicable diseases. This action, led in Cantabria by the Health Economics Group, the Clinical and Translational Research Group in Digestive Diseases, the Pediatrics Service of the Marqués de […]
IDIVAL organizes two days in which students from different fields and levels will face health challenges through innovation
The “Santander i-Days 2024” event is set to inspire students from all academic disciplines in the field of healthcare innovation. This year, the event will be held on two dates: on October 29 online, and on November 6 in person at the Téllez Plasencia Hall of the Marqués de Valdecilla University Hospital (HUMV).
The event, supported by the EIT Health program and organized by the Valdecilla Health Research Institute (IDIVAL), in collaboration with the University of Cantabria (UC), aims to foster the development of new skills in participants, giving them the opportunity to work in teams to address real challenges in the healthcare field.
During the first day of the event, which will be online, participants will be introduced to the challenges through team dynamics and role-play activities that will put them “in the patient’s shoes”. The session will continue with team building and a detailed explanation of the challenges, culminating in a concluding and closing session.
The second day, which will be held in person, will offer attendees the opportunity to delve into the transfer of results in healthcare, explore 3D printing applied to healthcare, and participate in group dynamics focused on creativity, ideation, and the development of prototypes. The day will also include parallel sessions and will conclude with the presentation of projects, selection of winning teams and the award ceremony.
The winning team in Santander will have the opportunity to compete in the Winners’ Event in Budapest, a meeting that will bring together the best students from across Europe for a final competition.
With the promise of acquiring new skills, receiving expert support and facing real challenges, the “Santander i-Days 2024” is a unique opportunity for students looking to make a difference in the field of healthcare innovation.
Interested parties can visit the event’s official website for more information and to register.


IDIVAL brings healthcare innovation closer to students
IDIVAL organizes two days in which students from different fields and levels will face health challenges through innovation The “Santander i-Days 2024” event is set to inspire students from all academic disciplines in the field of healthcare innovation. This year, the event will be held on two dates: on October 29 online, and on November […]
The rapid hepatological diagnosis is in the spotlight of IDIVAL researchers
IDIVAL Advances in One-Step Diagnosis for Determining MASH and Liver Fibrosis in High-Risk Populations The United European Gastroenterology Journal has just published an innovative study in the field of hepatology. The research, conducted by the Clinical and Translational Research Groupin Digestive Diesases at IDIVAL, led by Dr. Paula Iruzubieta and Dr. Javier Crespo, in collaboration […]
A global study that determines the frequency and type of genetic variants that cause the disease
Researchers from the neurodegenerative diseases group of IDIVAL and the Neurology Service of HUMV, led by Dr. Jon Infante, have participated in an international study carried out in 16 countries and on 12,580 patients with Parkinson’s disease.
Although the cause of Parkinson’s disease is not genetic in most cases, it is estimated that around 10% may be caused by mutations in various genes. However, the knowledge we have regarding the spectrum and frequency of different genetic mutations globally is limited and biased. At a time when the first clinical trials with therapies targeting specific genetic subtypes of Parkinson’s have been initiated, a major obstacle to the conduct of these trials is that many Parkinson’s patients do not know whether their disease is caused by any of these genes.
The Rostock Parkinson’s disease study (ROPAD), published in the prestigious journal Brain, is an observational clinical study aimed at determining the frequency and spectrum of genetic variants contributing to PD in a large international cohort. Variants in 50 genes were investigated in a group of 12,580 Parkinson’s disease patients from 16 countries using a next-generation sequencing panel. In total, in 1864 participants (14.8%) some gene mutation was identified, the most frequent being in the GBA1 (10.4%), LRRK2 (2.9%), PRKN (0.9%), SNCA (0.2%) or PINK1 (0.1%) genes or a combination of mutations in two genes in two genes (∼0.2%). In patients with age of onset less than 50 years the frequency of mutations was 19.5% and in those who also had a family history it rose to 26.9%.
In the emerging era of gene-targeted clinical trials, the finding that ∼15% of patients harbor potentially actionable genetic variants offers an important perspective to affected individuals and their families, and underscores the need for genetic testing in patients with Parkinson’s disease.
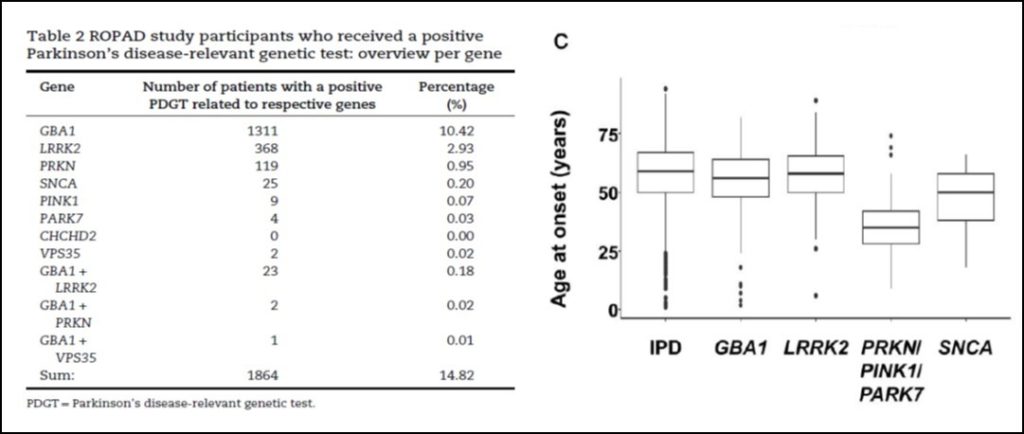
The neurodegenerative diseases group of IDIVAL has a long history of research in the field of Parkinson’s disease genetics, having contributed to define the genetic epidemiology of a genetic subtype of Parkinson’s disease, associated with variants in the LRRK2 gene, particularly frequent in Cantabria. Through different studies this group has contributed to the identification of biomarkers of the presymptomatic stages of the disease.
Reference:
Westenberger A, Skrahina V, Usnich T, Beetz C, Vollstedt EJ, Laabs BH, Paul JJ, Curado F, Skobalj S, Gaber H, Olmedillas M, Bogdanovic X, Ameziane N, Schell N, Aasly JO, Afshari M, Agarwal P, Aldred J, Alonso-Frech F, Anderson R, Araújo R, Arkadir D, Avenali M, Balal M, Benizri S, Bette S, Bhatia P, Bonello M, Braga-Neto P, Brauneis S, Cardoso FEC, Cavallieri F, Classen J, Cohen L, Coletta D, Crosiers D, Cullufi P, Dashtipour K, Demirkiran M, de Carvalho Aguiar P, De Rosa A, Djaldetti R, Dogu O, Dos Santos Ghilardi MG, Eggers C, Elibol B, Ellenbogen A, Ertan S, Fabiani G, Falkenburger BH, Farrow S, Fay-Karmon T, Ferencz GJ, Fonoff ET, Fragoso YD, Genç G, Gorospe A, Grandas F, Gruber D, Gudesblatt M, Gurevich T, Hagenah J, Hanagasi HA, Hassin-Baer S, Hauser RA, Hernández-Vara J, Herting B, Hinson VK, Hogg E, Hu MT, Hummelgen E, Hussey K, Infante J, Isaacson SH, Jauma S, Koleva-Alazeh N, Kuhlenbäumer G, Kühn A, Litvan I, López-Manzanares L, Luxmore M, Manandhar S, Marcaud V, Markopoulou K, Marras C, McKenzie M, Matarazzo M, Merello M, Mollenhauer B, Morgan JC, Mullin S, Musacchio T, Myers B, Negrotti A, Nieves A, Nitsan Z, Oskooilar N, Öztop-Çakmak Ö, Pal G, Pavese N, Percesepe A, Piccoli T, Pinto de Souza C, Prell T, Pulera M, Raw J, Reetz K, Reiner J, Rosenberg D, Ruiz-Lopez M, Ruiz Martinez J, Sammler E, Santos-Lobato BL, Saunders-Pullman R, Schlesinger I, Schofield CM, Schumacher-Schuh AF, Scott B, Sesar Á, Shafer SJ, Sheridan R, Silverdale M, Sophia R, Spitz M, Stathis P, Stocchi F, Tagliati M, Tai YF, Terwecoren A, Thonke S, Tönges L, Toschi G, Tumas V, Urban PP, Vacca L, Vandenberghe W, Valente EM, Valzania F, Vela-Desojo L, Weill C, Weise D, Wojcieszek J, Wolz M, Yahalom G, Yalcin-Cakmakli G, Zittel S, Zlotnik Y, Kandaswamy KK, Balck A, Hanssen H, Borsche M, Lange LM, Csoti I, Lohmann K, Kasten M, Brüggemann N, Rolfs A, Klein C, Bauer P. Relevance of genetic testing in the gene-targeted trial era: the Rostock Parkinson’s disease study. Brain. 2024 Aug 1;147(8):2652-2667. doi: 10.1093/brain/awae188. PMID: 39087914; PMCID: PMC11292909.
Cover photo: IDIVAL neurodegenerative diseases group.
IDIVAL’s neurodegenerative diseases group participates in a study that defines the genetic epidemiology of Parkinson’s disease.
A global study that determines the frequency and type of genetic variants that cause the disease Researchers from the neurodegenerative diseases group of IDIVAL and the Neurology Service of HUMV, led by Dr. Jon Infante, have participated in an international study carried out in 16 countries and on 12,580 patients with Parkinson’s disease. Although the […]



















